Project: to produce tag-free, soluble GITR/GITRL complex assembled in cell culture
Step I: Establishment of stable cell line producing recombinant GITR
Stable chinese hamster ovary cell line producing GITR-Fc chimera has been developed using standard protocols established in Recepton.
As a part of our first project, we have developed platform for testing new, potentially therapeutic entities targeting immune checkpoint receptors. The platform required reliable cell line development methods and production of proteins with consistent characteristics. Our commerically available products and services reflect those needs.
Significant elements of our early-stage cell line development are:
- optimized workflow, comprising outsourcing gene synthesis to reliable contractors and well-established, flexible methods of molecular cloning. We are able to deliver final product produced in stable cell line as fast as 3 months, from order to delivery, depending on required specification.
- proprietary pREC plasmid system, enabling robust expression of multiple proteins or multiple subunits of single protein from single culture.
GITR-Fc construct has been cloned into pREC1G plasmid, which facilitates G418 resistance.
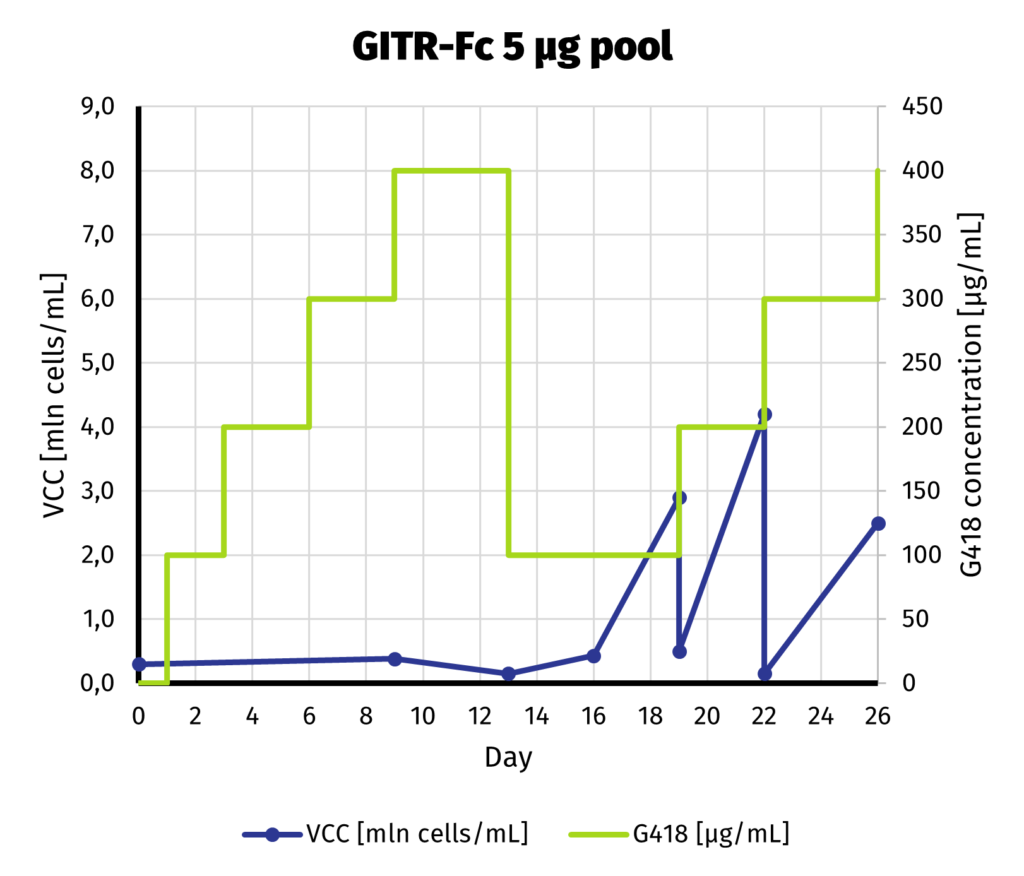
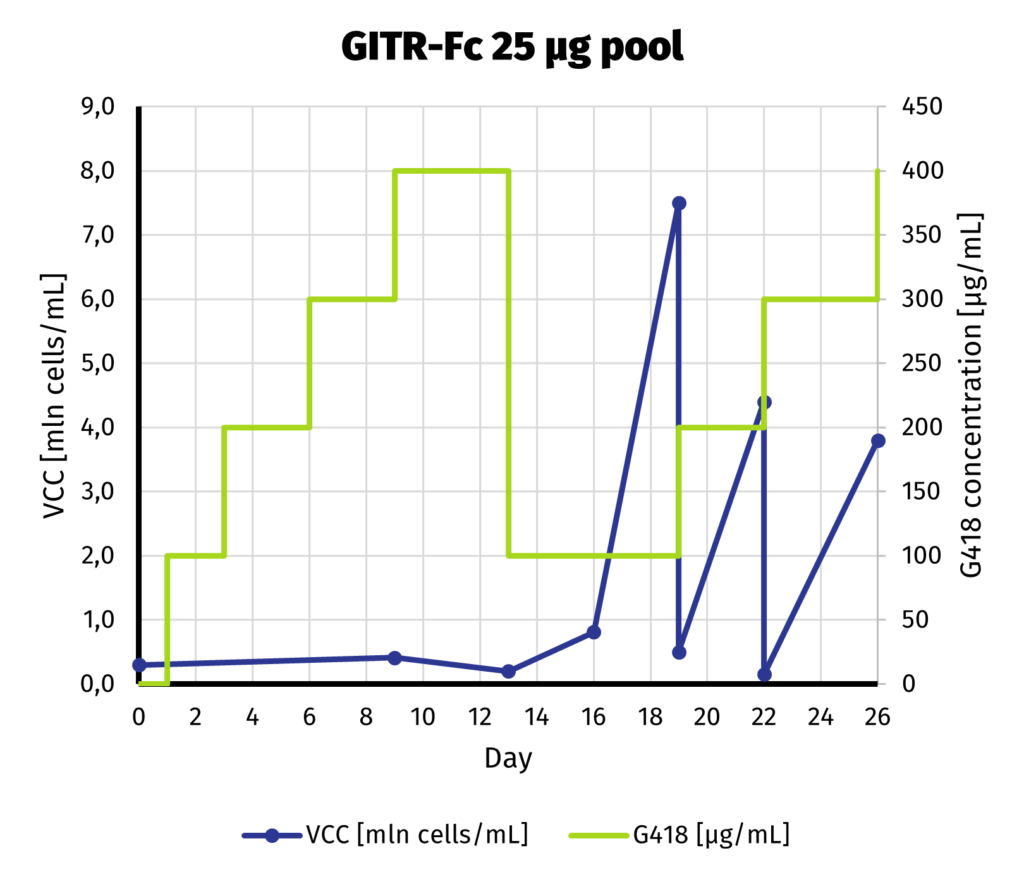
Suspension-adapted CHO K1 cells have been electroporated with 5 and 25 µg of linearized DNA, using square wave protocol. We optimized the protocol with DoE approach, to yield as much protein as possible from each electroporation. Process of G418 selection is presented on charts below.
We can safely assume that the cell line is established as soon as day 19 from electroporation. Our previous experiments show that further increase of selection pressure (up to 1000 µg/mL of G418) after initial adaptation does not lead to increase in productivity.
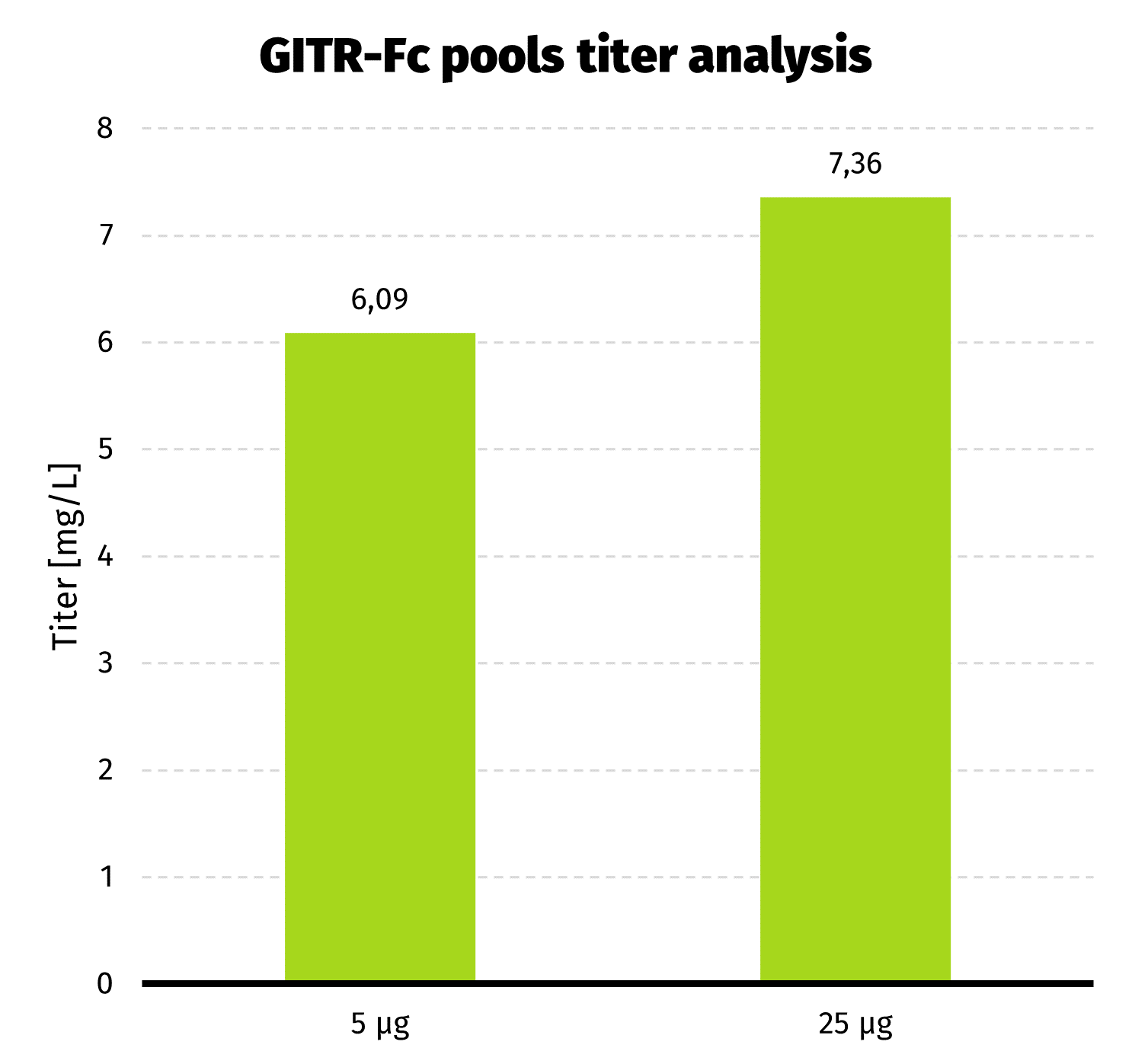
We continue the culture from day 19 for another week to assess productivity by Protein A-UPLC (depending on available tags). If faster delivery time of clonal cell line is desirable, single cell cloning may be started before productivity assessment has been carried out. Based on results from both pools, 25 µg pool has been selected for establishment of clonal cell line.
6 96-well plates have been seeded with 0,8 cell/well, using limiting dilutions method. After 14 days we carried out productivity screening in four steps:
- initial productivity screening by high-throughput immunoenzymatic methods, using anti-human IgG antibodies to distinguish between producers and non-producers;
- semi-quantitative western blot on 20 of the most promising clones to further narrow down number of clones and check size of produced protein
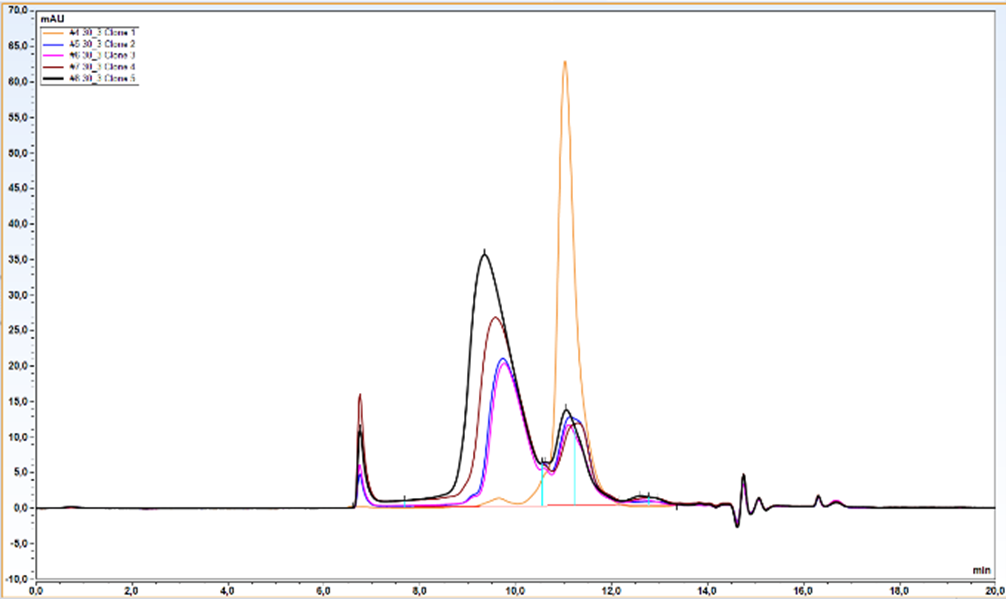
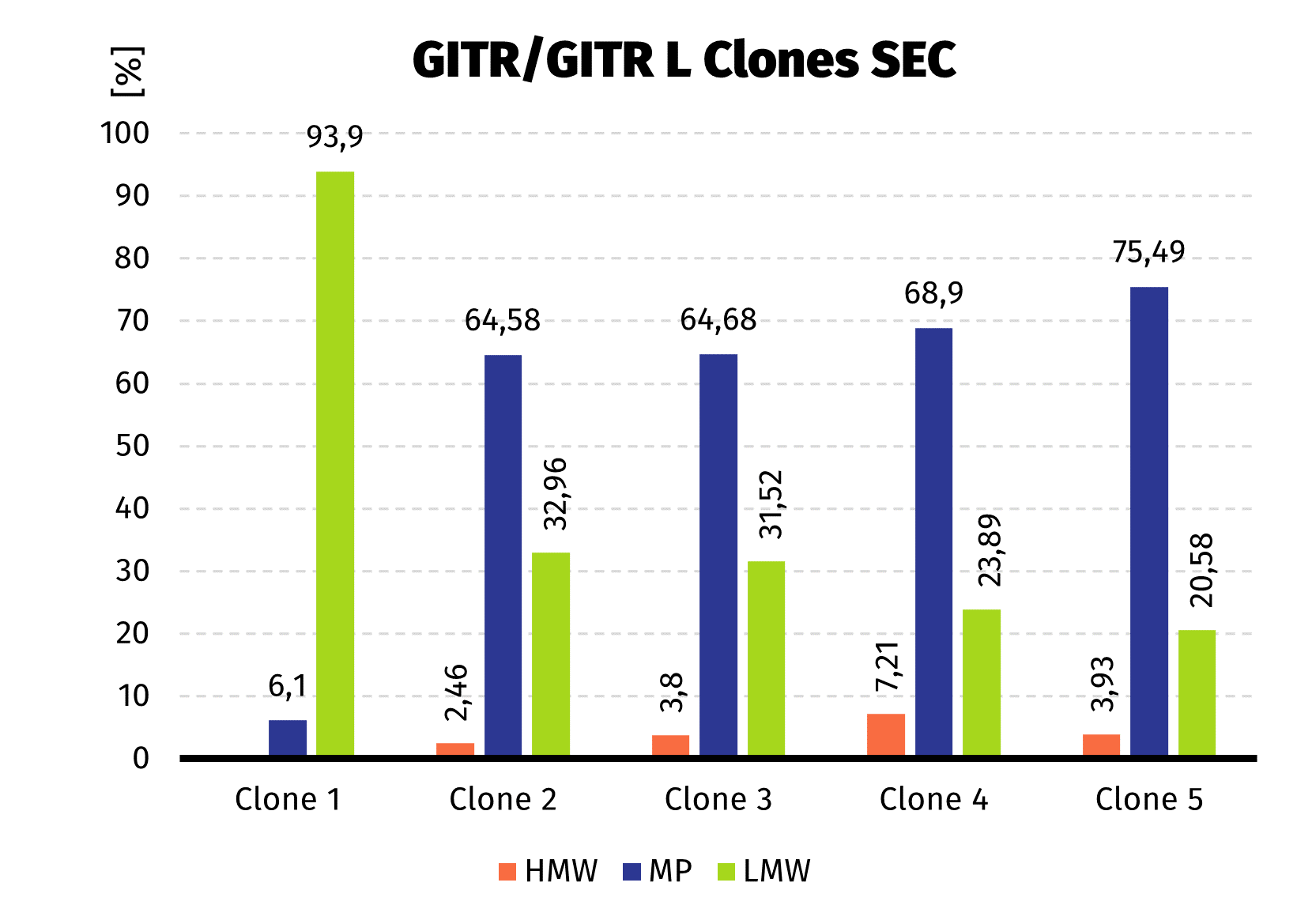
- 5 clones selected by western blot were analyzed quantitatively by Protein A-UPLC
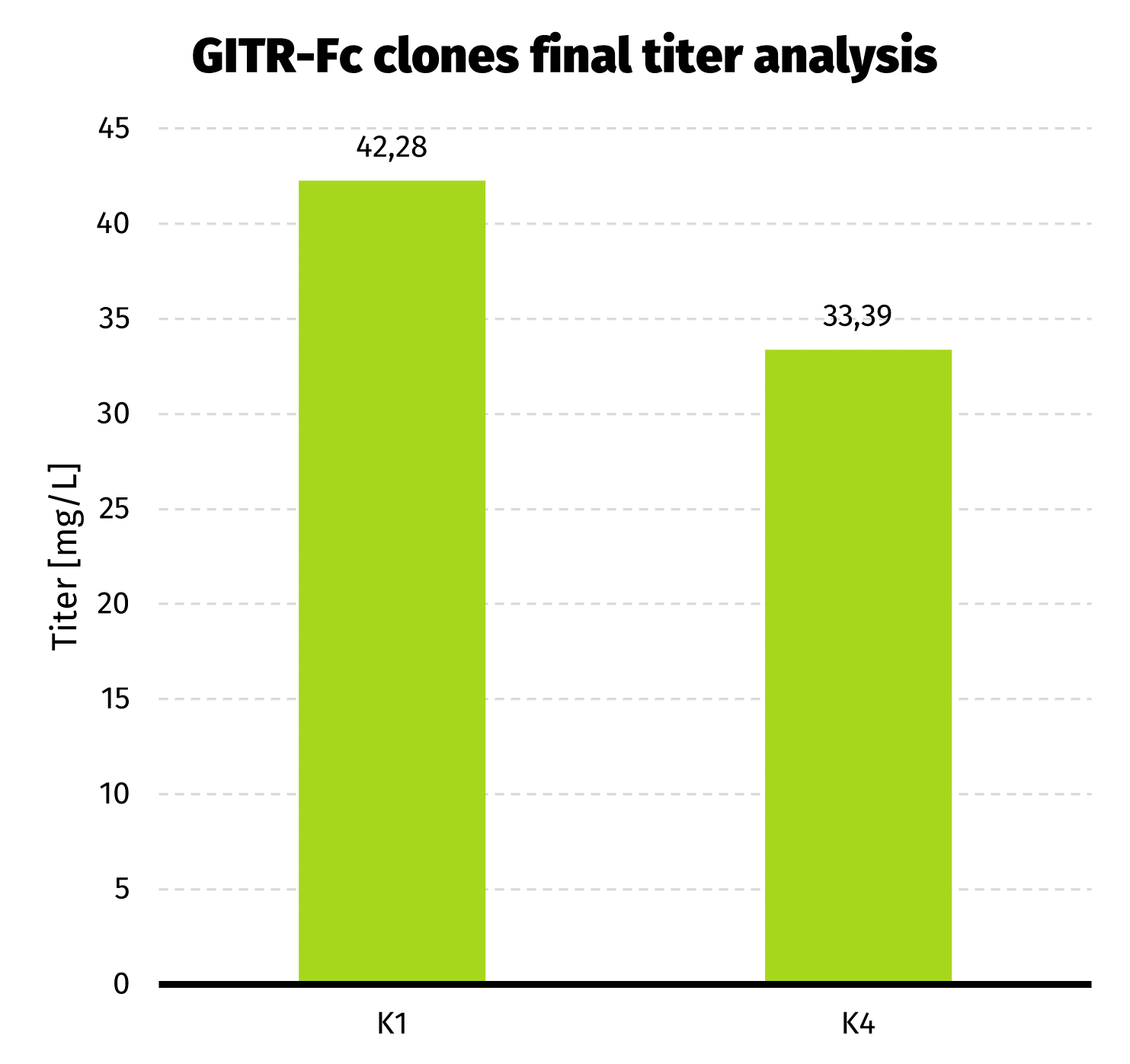
- 2 clones with best productivity were passaged to 30 mL cultures, and after 7 days of batch culture final titer assessment has been performed.
We observed almost 6-fold increase in productivity compared to polyclonal cell pool. Results from UPLC analysis are presented on charts below.
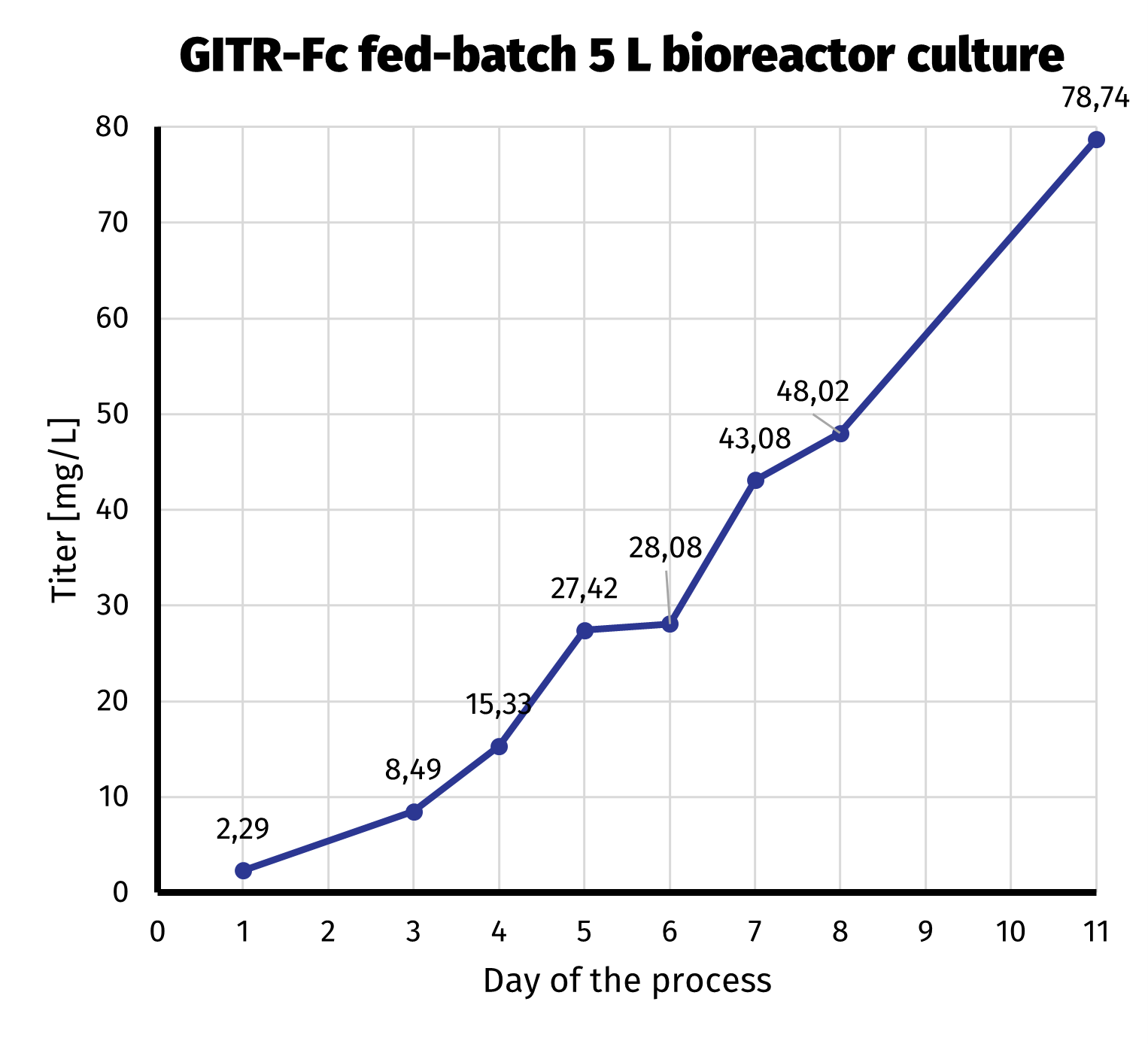
After the clonal cell line had been established, we started 5 L bioreactor culture in order to further increase productivity of GITR-Fc. We routinely use fed-batch protocols in bioreactor productions, as the yield is significantly higher when compared to batch cultures. The amount of labor and equipment necessary is on the other hand not that demanding. Day-by-day productivity analysis from bioreactor culture is presented on the chart below.
Step II: Preparation of tagless GITRL construct
Whereas establishing stable, clonal cell lines is something we do routinely, we are also open for more custom and challanging projects. Co-expression of GITRL with GITR in a single culture was one of those projects.
We prepared tagless GITRL construct by PCR, using our previously available GITRL-Fc chimera genetic construct as a matrix. It has been cloned into pREC3H plasmid, which facilitates Hygromycin B resistance.
We introduced obtained GITRL-pREC3H plasmid into previously established clonal cell line producing GITR-Fc chimera protein. Following Hygromycin B selection (along with constant G418 concentration), polyclonal cell pool has been obtained.
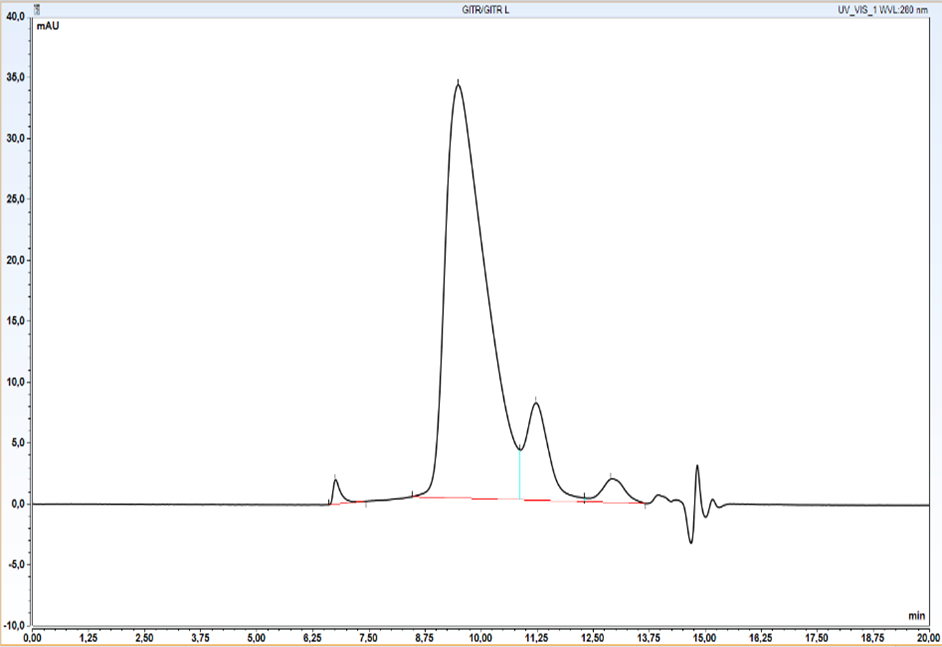
Initial qualitative analysis as well as semi-quantitative productivity assessment has been carried out at this stage by HPLC SEC. We wanted to ensure as early as possible that protein of appropriate size was being produced.
Single cell cloning and selection with immunoenzymatic methods resulted in selection of robust lead clone. All analysis were performed using specific anti-GITRL antibodies. Lead clone has been further expanded for production in 5 L bioreactor.
Step III: Large-scale production in bioreactor
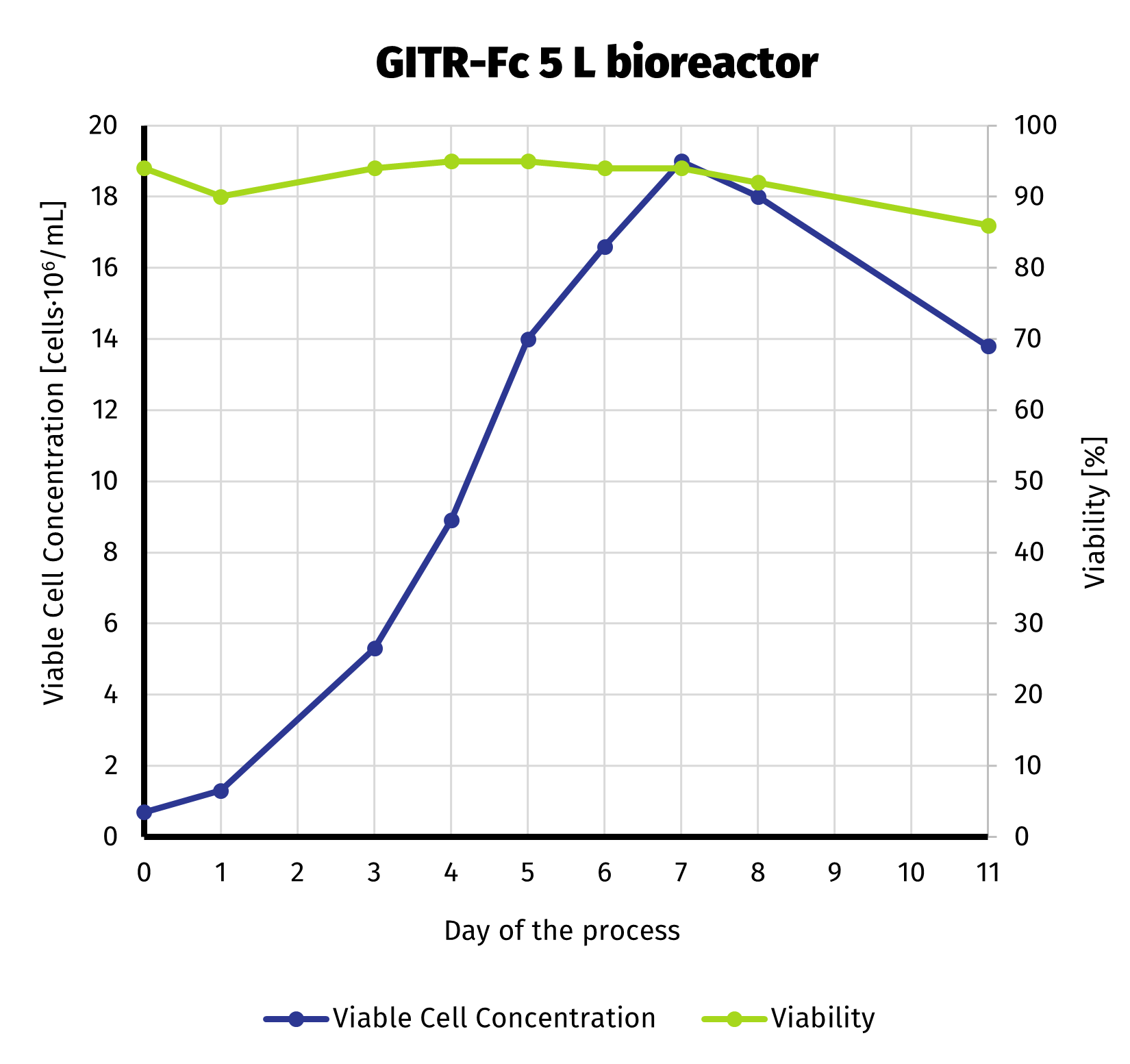
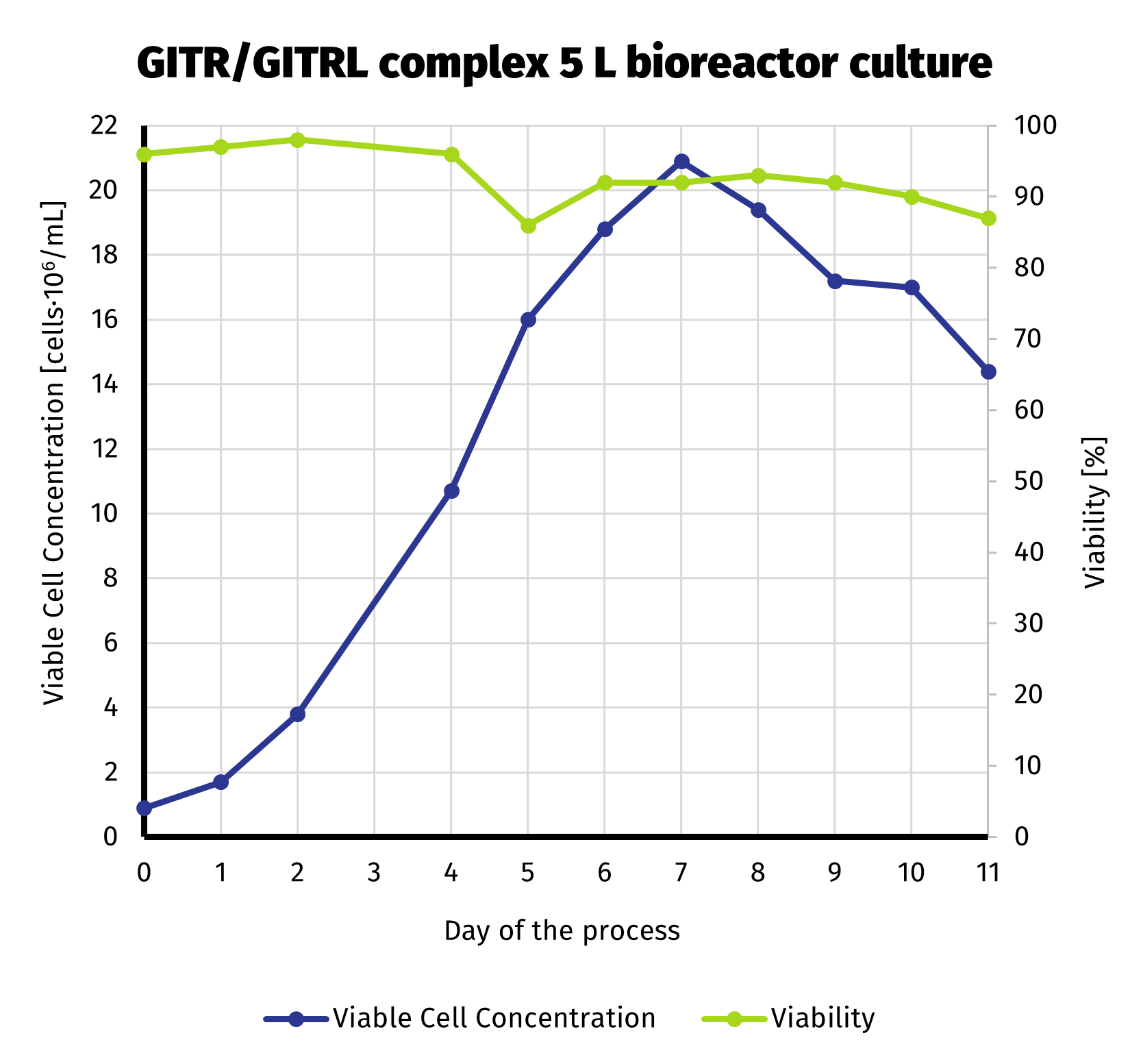
To deliver required amount of protein, we scaled up the production culture to 5 L. Production stage has been carried out in bioreactor. With more control over the process compared to shake flasks, we were able to extend production up to 11 days. Despite noticeable decline in viable cell number starting on day 8, culture still exhibited quite high viability until the end of the run (>85%). VCC and cell viability is presened on the chart below.
Step IV: Purification & tag digestion
After culture had been harvested through centrifugation and filtration, obtained filtrate was purified further by three steps: Affinity Chromatography (protein A), Fc-tag digestion and Size Exclusion Chromatography.
Affinity chromatography: Harvest was loaded onto protein A resin, which further was washed with phosphate buffer, TRIS buffer without CaCl2 followed by TRIS buffer with CaCl2 pH 8.0.
Fc-tag digestion: GITR/GITR L complex bound to the resin was incubated with Factor Xa, after digestion a flow through comprising of GITR/GITR L complex without Fc-tag was collected.
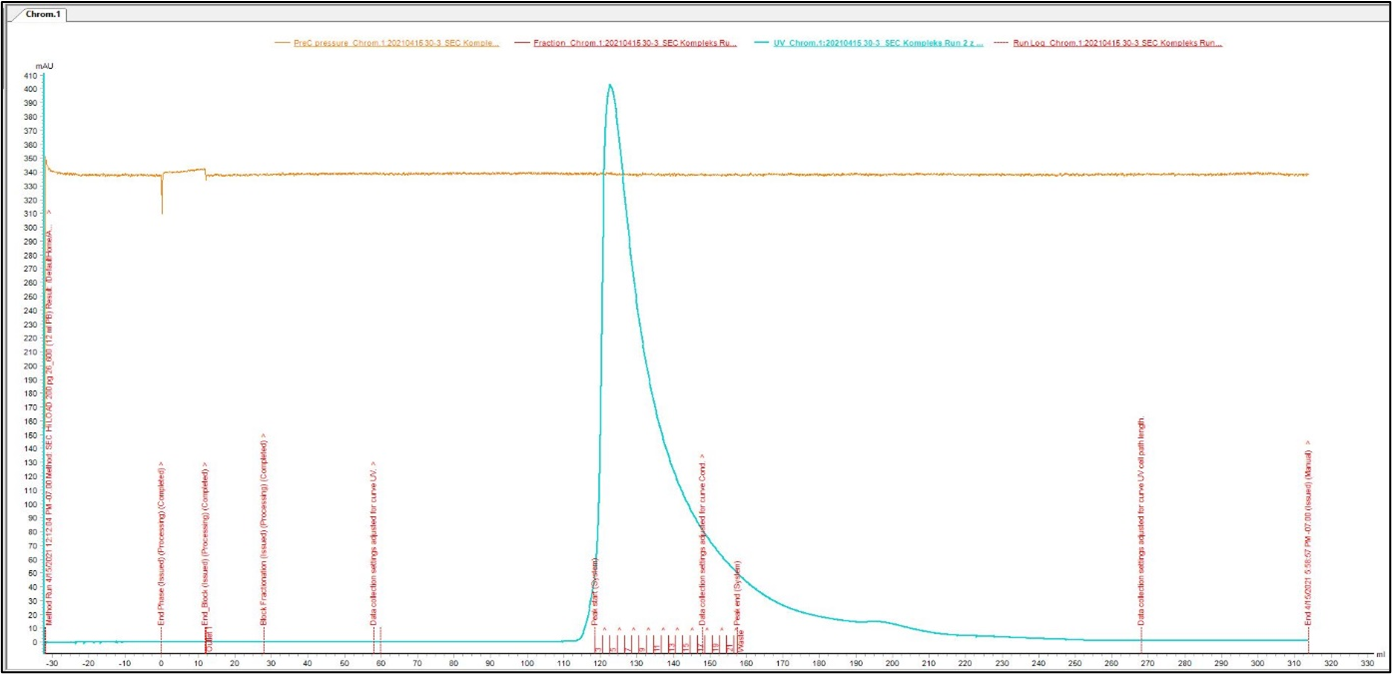
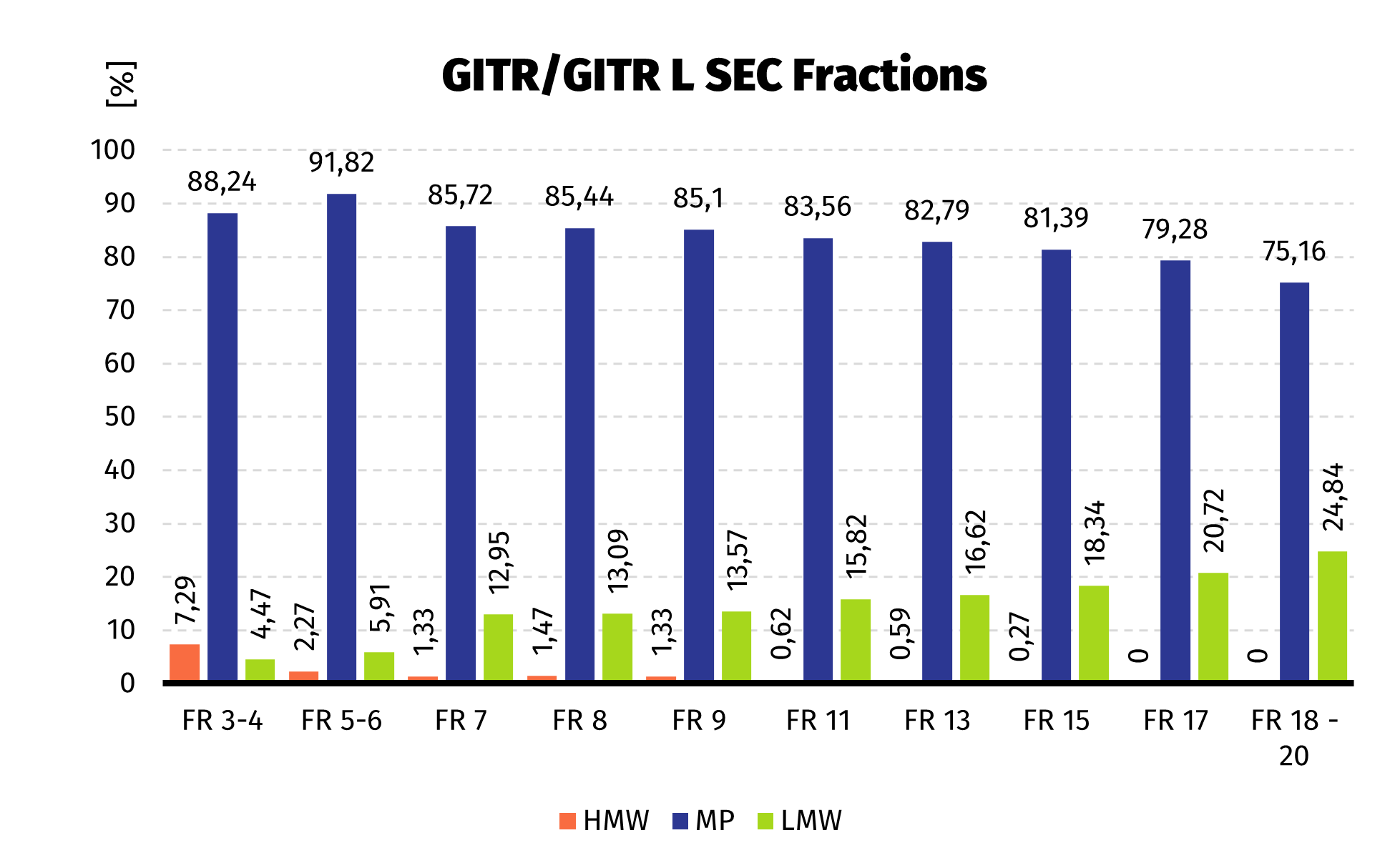
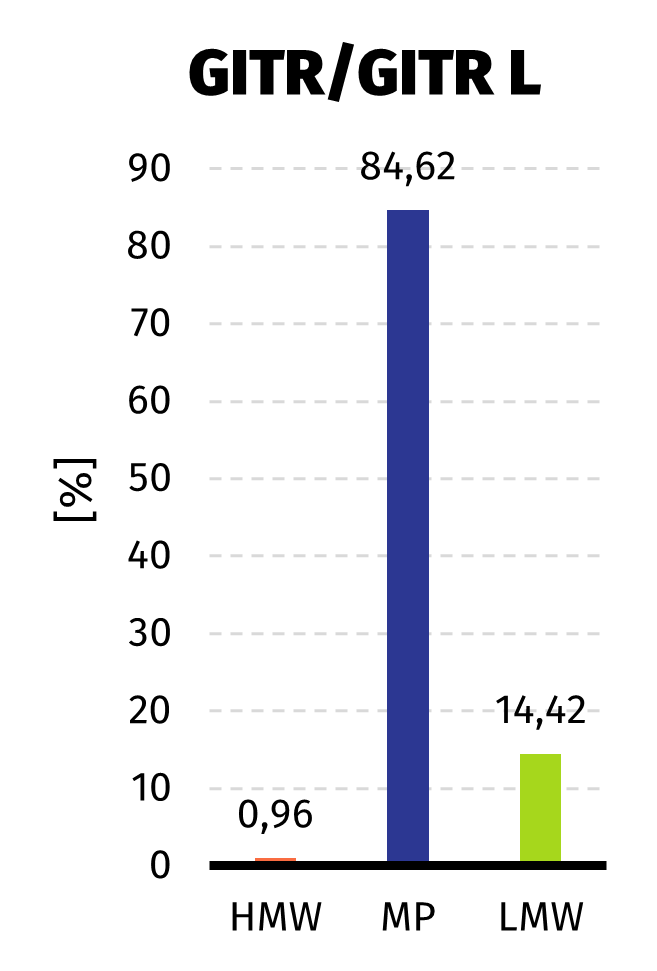
Size Exclusion Chromatography: Protein complex was loaded onto Superdex 200 pg column and eluted in 19 fractions. The fractions were further analysed on HPLC SEC, to choose the most homogenous protein.
Step V: Quality Assurance
Quality of the obtained protein has been analyzed with SDS-PAGE, size exclusion chromatography (size and purity) and immunoenzymatic methods using anti-GITRL antibodies (confirmation of GITRL identity). Since affinity tag was present only on one protein, analysis results confirmed that proteins were co-expressed and purified as expected.